Energy production system -Universal machinery in life-
We all use a lot of energy to maintain our life.
A heart beats 100 thousand times every day, requires a lot of energy. However, the storage of ATP in the heart at one time can last only 10 seconds, suggesting our heart needs continuous production of ATP in mitochondria.
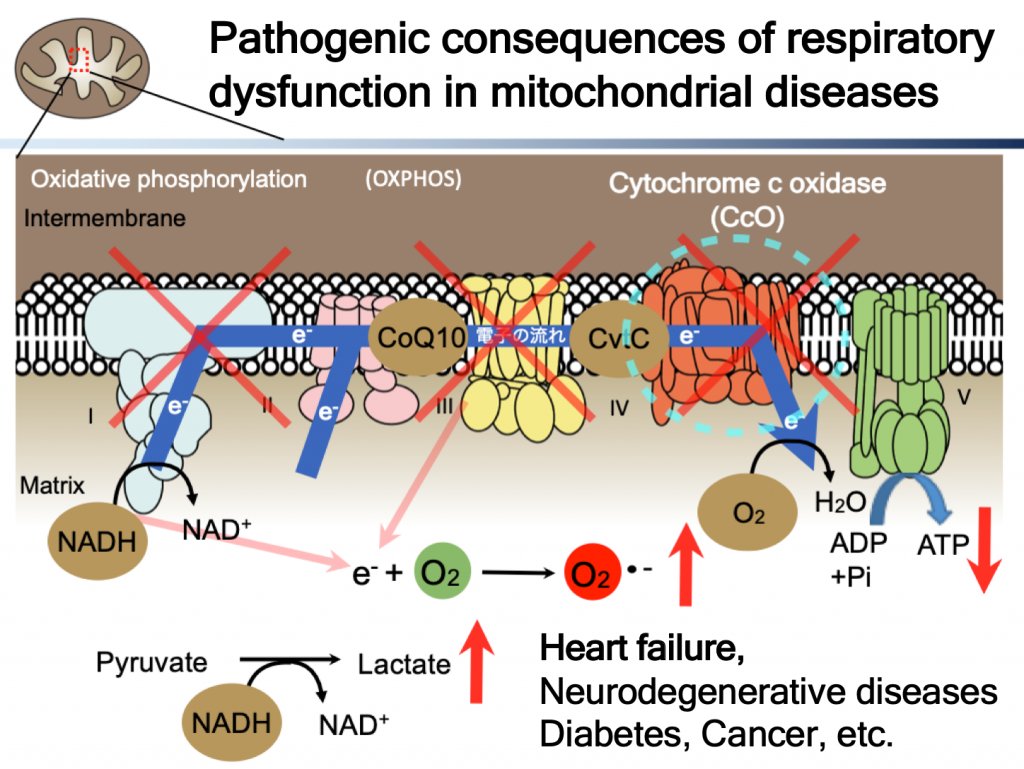
In mitochondria, ATP is produced by electron transfer followed by oxidative phosphorylation. There are five protein complexes at the inner mitochondrial membrane. In ETC, what these protein complexes do is transfer electrons from NADH through coenzyme Q10 (ubiquinol), cytochrome c and finally to oxygen. While doing so, protons are transferred from matrix to intermembrane spaces in order to create/maintain proton gradient (energy) by use of the difference of redox potential (energy) between NADH and molecular oxygen (O2). This proton gradient is motive force for rotatory ATP synthase (ADP to ATP), final coupling step between electrical and chemical energy.
Dysfunction in electron transfer and oxidative phosphorylation causes mitochondrial diseases. Pathogenic consequences of respiratory dysfunction are, of course, lack of ATP, oxidative stress, because electrons are transferred from excess reducing equivalent directly to molecular oxygen, and lactic acidosis for compensatory increased glycolysis. all of them contribute to pathogenesis, and current treatment for this disease is not good enough, none of the treatment has been approved by FDA so far. There are huge unmet medical needs.
As the primary cause of mitochondrial disease is dysfunction in respiratory chain, we wanted to improve electron transfer in these patients, if we can do that, it can mitigate all the pathogenic consequences.
Cytochrome c oxidase
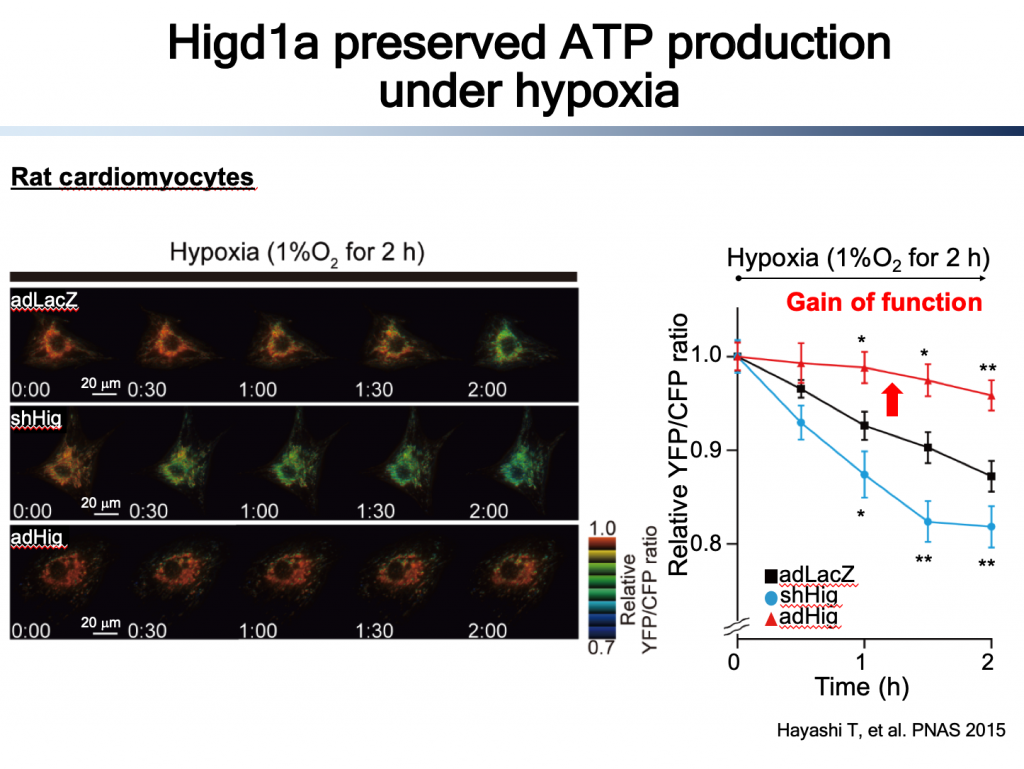
CcO is the terminal enzyme of electron transport chain, and the only enzyme that uses oxygen. Most of oxygen which are taken into cells are consumed by CcO. We have been focusing CcO because we found an endogenous CcO activator, Higd1a (1). Higd1a is induced by hypoxia, Hind1a binds directly to CcO, leading to structural change around heme a, resulting in increase in enzymatic activity of CcO and ultimately ATP production (1, 2). These findings confirmed that CcO can be a rate-limiting enzyme in ETC/OXPHOS in certain circumstances, most importantly, the robust energy producing system can be modulated by the CcO activity.
These findings suggested that CcO may be allosterically modulated by small compound with possibility of therapeutic application. Therefore, we set up small compound library screening and performed more than 200 thousand cpds, and found several hit compounds. We confirmed hit compounds increased CcO activity and ATP production in the cells with normal respiratory function and also in different cellular models of mitochondrial disease.
We are currently working on further development of these CcO activator as a potential therapeutic agent for mitochondrial diseases or secondary respiratory dysfunction including heart failure.
【References】
1. Hayashi T, et al. Proc Natl Acad Sci U S A. 2015 Feb 3;112(5):1553-8.
2. Nagao T, et al. FASEB J. 2020 in press
